The QM solution for your University Hospital
The QM solution for your University Hospital
- Document control according to standards
- Simple process automation
- Easy operation & secure access
Our software solutions can already be found in over two-thirds of German university hospitals
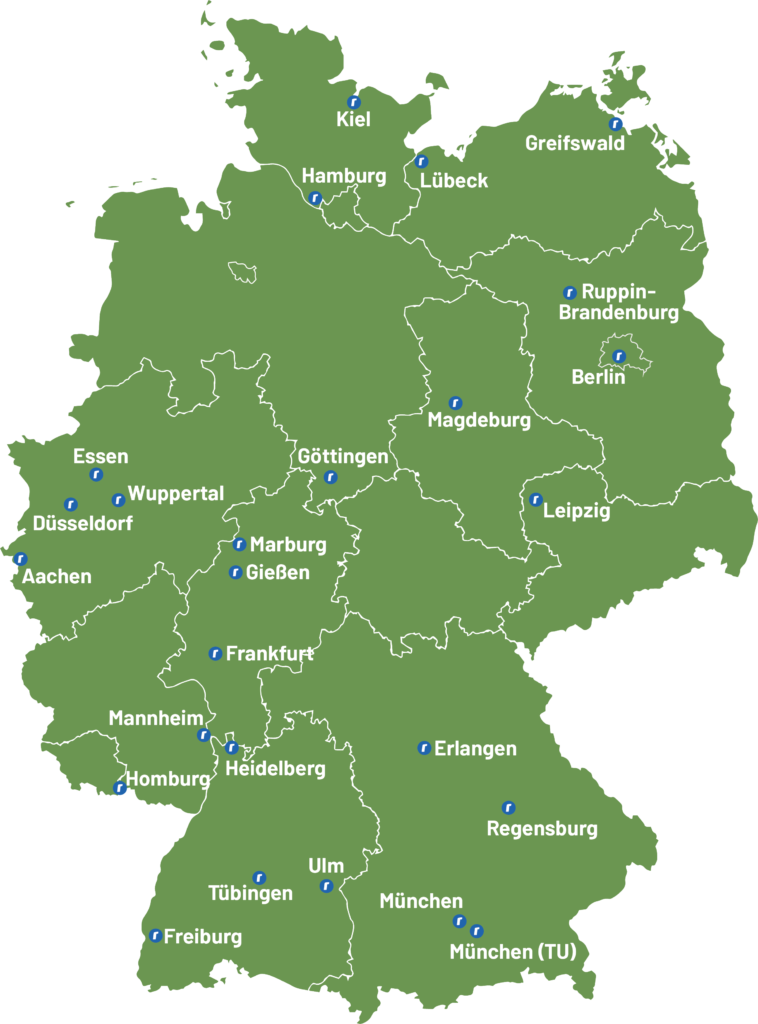
An excerpt of our customers









roXtra supports you with the following solutions:
roXtra QM Software in University Hospitals
A university hospital is not only a place of interdisciplinary, medical treatment, but also an institution for research, training and teaching. Tomorrow's medical professionals take their first steps here and new methods for diagnosing and treating patients are developed. This makes it all the more important to have a functioning Quality Management System (QMS) that helps to prepare the path for innovation while still ensuring patient safety and employee orientation.
More than half of the German university hospitals trust roXtra – the market leader in your sector.
roXtra supports you in uniting medical treatment as well as research and teaching in your institution, also with regard to QM documentation. Work entirely according to the motto "everything under one roof" or separate the individual locations and specialist areas from each other by means of area administrations.
Institutes, clinics, doctors and laboratory areas have different requirements for QM documentation. Thanks to the flexibility of roXtra, we can offer you a solution that is both user-friendly and compliant. In doing so, roXtra fulfils common standard specifications (for example ISO 9001, KTQ and ISO 13485) as well as the regulations in the GxP-regulated environment and for operators of critical infrastructure (KRITIS). Here you will find an overview of how the QM software roXtra implements the requirements of regulations such as 21 CFR Part 11, the EU EMP Guideline (Annex 11) and the guidelines of the German Medical Association (Rili-BÄK). In addition, we are happy to support you during implementation, validation and throughout the entire system operation.
We configure the software according to your ideas, allowing you to implement your corporate design as well as regulatory and technical requirements. Use the software solution to design your individual workflows, document templates and folder structures and store your retention periods, metadata fields and password guidelines. With roXtra you are ideally prepared for internal and external audits as well as certification and re-certification.
With the roXtra Document Workflow you keep an overview of your documentation – from creation to archiving, locally as well as location-independent. Whether you are in the hospital, in the lecture hall, in the medical care center or in the office, with roXtra you always have access to the valid version of your documents.
The review and approval of, for example, new and revised work and process instructions becomes structured, comprehensible and transparent. During certification, you can quickly and easily provide your auditor with an overview of all relevant documents, including important meta information (e.g. resubmission date, status and revision).

Optimize and automate your Process Management with the module roXtra Processesand thus, bring your applications and forms digitally "to life". From applications for leave, CAPA notifications, quality assurance to change management and the implementation of continuous improvement actions – you can easily map and execute your individual forms and processes.
To visualize your processes, e.g. in the form of flowcharts, we offer you with the Flowchart-Designer the ideal alternative to visualization programs such as Microsoft Visio. Create concise graphics and use them to illustrate, for example, the structure of your EFQM model or critical control points, that require special attention with regard to patient safety.

With roXtra risks you can reduce the amount of work required for your cross-company risk management. Identify and manage potential risks centrally for an effective, qualitative risk analysis in the university medicine environment. roXtra supports the common standards and regulations (e.g. ISO 31000 or ONR 49001, ISO 15224, ISO 9001 and ISO 27001 / IEC 8001).
In healthcare, patient safety is the top priority. Risks cannot be completely eliminated - but effective risk management supports the avoidance, reduction, optimization and transfer of risks. Many regulations therefore require a risk management system to support ongoing operations. In this way, you can prevent and avoid clinical risks (e.g. inadequate hygiene, mistaken procedures, incorrect diagnoses, etc.) as well as non-clinical, business risks (e.g. the loss of customers and thus a financial risk) in the future.

Audits are an essential part of your work as a quality manager and internal auditor?
You know, a systematicaudit management is your most powerful tool as an auditor?
Increase the effectiveness and quality of your own work with the roXtra Audits module. The software supports you in auditing management systems according to the specifications such as those of ISO 19011.
Thanks to roXtra Audits, you work with a system and access the questionnaire for conducting audits regardless of location, even offline. In this way, you increase internal efficiency and diligence when conducting audits. At the same time, your team and you gain an overview of the status of past, current or pre-planned audits.
Focus on identifying internal nonconformities and areas for improvement. Defined immediate, corrective and preventive actions are documented and started directly in the context of the audit. No matter whether DIN EN ISO 9001, ISO 13485, DIN EN ISO/IEC 17025 or ISO 14001: roXtra helps you to meet the various requirements of current standards with flying colors in order to do a really good job.

Our software solutions support you in daily system operation as well as during certification and re-certification of your Quality Management system. With roXtra, the implementation of a multitude of standards and regulations becomes a breeze.
- KTQ Certification: Cooperation for Transparency and Quality in the Healthcare Sector
- DIN EN ISO 9000 and DIN EN ISO 9001 Quality Management systems - Requirements
- DIN EN 15224 Quality management systems for the health care sector
- DIN EN ISO 31000 - Guidelines for risk management
- or ONR 49000 / ONR 49001 - Austrian risk management standard
- DIN EN ISO 13485 Medical devices Quality Management systems - Requirements for regulatory purposes
- Requirements for operators of critical infrastructures (CRITIS)
- Claims of the Federal Joint Committee (G-BA), the German Hospital Federation e.V. (Deutsche Krankenhausgesellschaft – DKG) as well as the German Nursing Council e.V. (DPR)
- ISO 27001 - Information Security Management System (ISMS) / IT Security
- or IEC 80001 standard for risk management in networked medical technology
- KonTraG - Law on Control and Transparency in the Corporate Sector (Corporate Governance)
- Section 91 (2) AktG - Establishment of a risk management system (RMS) for listed companies

success stories
We can tell you a lot of things...
...that's why we give our users a voice. In this way, you receive first-hand reports of experiences directly from practice. See for yourself and learn more about the use of roXtra in different areas. Would you like to learn more about how university hospitals have implemented roXtra in their quality management? Do you have questions about the design of an institution's internal quality management system (QMS), the validation of computer-based systems or the requirements of KTQ, ISO 13485, G-BA and Co? In our blog, we regularly inform you about topics related to quality management, its implementation in healthcare, as well as examples of its application in hospitals, in quality assurance and actions for continuous improvement.
- In the service of patientsTogether with the Rennbahnklinik, we have published a user report in "QZ - Quality and Reliability". Read the full article here.
- Smart sensor solutions need smart document controlTogether with our customer Sensirion AG, we have published a case study in the trade journal MQ Management und Qualität. Read the complete article here.
- Mental health also needs processesTogether with our customer Dr. Fontheim - Mental Health, we have published a case study in the trade journal QZ Qualität und Zuverlässigkeit. Read the full article here.
Experience roXtra live
We will show you roXtra in a free and non-binding online presentation.




